
APPLICATIONS
Os Encat™
Dihydroxylation Reaction
Osmium tetroxide catalyses the formation of 1,2-diols from the corresponding olefins in the presence of a co-oxidant. The use of catalytic amounts of Os EnCat™ together with stoichiometric amounts of a secondary oxidant converts various alkyl and aryl olefins to the corresponding diols.
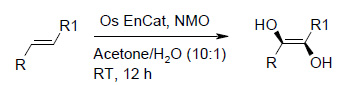
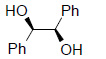
Table 1 below summarises some of the substrates that have been successfully converted to their respective diols with Os EnCat™ 40.
Table 1. Dihydroxylation Reaction of Diverse Olefins with Os EnCat™ 40
| Substrate | Product | Yield³/% |
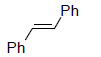 |
 |
84 |
 |
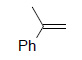 |
90 |
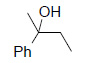 |
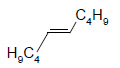 |
84 |
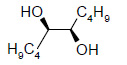 |
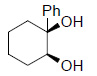
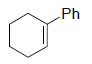 |
82 |
 |
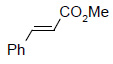 |
85 |
Reagents and conditions: Olefin, NMO (1.5 mmol), Os EnCat™ 40 (5 mol%), acetone/H2O (10:1), rt, 12 h; based on isolated yields
Following filtration of the catalyst, aqueous extraction and concentration of organic phase crude products typically contained 10-40 ppm osmium as measured by ICP.
The utility of Os EnCat™ 40 has also been extended to include asymmetric dihydroxylation (AD) reactions. Performing reactions under the 3 - Sharpless conditions with presence of hydroquinidine 1,4-phthalazinediyl (DHQD)2PHAL ligand, stereoselectivity may be introduced.
| Substrate | Product | Yield/ % | ee/ % |
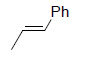 |
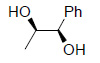 |
97 | 94 |
Oxidative Cleavage of Glycols
Osmium tetroxide is also used as a catalyst for the generation of carbonyl compounds from olefins by oxidative cleavage using sodium periodate (NaIO4).
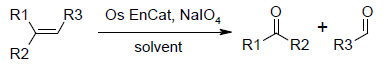
Os EnCat™ 40 has also shown to be an effective catalyst for these transformations, giving the desired carbonyl compounds in good yields under mild reaction conditions.
Oxidative Cleavage
| Substrate | Product | Yield% |
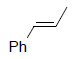 |
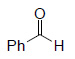 |
92³ |
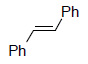 |
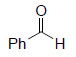 |
99³ |
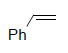 |
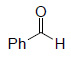 |
79³ |
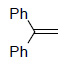 |
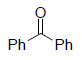 |
99³ |
Os EnCat™ 40 (2 mol%), THF/H2O (2:1), rt, 1-8h; a Isolated and identified as the phenyl hydrazone




