

APPLICATIONS
Ni (0) EnCat™
Nitrile Reductions
Atorvastatin Intermediate:
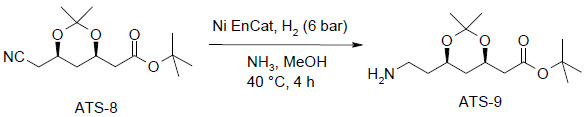
- Ni EnCat™ (25% wt) added to ATS-8 (0.8 mmol) in NH3/MeOH
- 6 bar Hydrogen, 40°C, 4.0 h
- 100% conversion
- 5 recycles without top-up
- Beads recovered undamaged and hence potential to recycle further
- Low levels of Nickel leaching – 14-20ppm
| Run | Ni loading wt(%) | Isolated Yield (%) |
| 1 | 25 | 95 |
| 2 | 25 | 96 |
| 3 | 25 | 96 |
| 4 | 25 | 96 |
| 5 | 25 | 96 |
Dextromethorphan Intermediate:
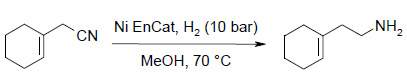
| Nitrile (g) | MeOH (mL) | Aq. NH3 (mL) | Ni EnCat™, dry weight (g) | Time (h) | GC purity (%) |
| 75 | 300 | 88 | 30 | 1 | 87 |
| 75 | 300 | 88 | Recovered from Cycle 1 | 1 | 86 |
| 75 | 300 | 88 | Recovered from Cycle 2 | 1.5 | 85 |
Conclusion: Ni EnCat™ can be recycled twice (3 cycles) with consistent purity.
A certain impurity in the crude reaction mixture when using a conventional catalyst was not observed with Ni EnCat™, leading to easier purification by fractionation / distillation and increased yields.
Double Bond Reaction
Stability to Sulphur containing substances:
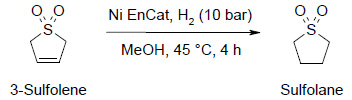
| Run | Ni loading wt% | Product (%) |
| 1 | 1.4 | 100 |
| 2 | 1.4 | 96 |
| 3 | 1.4 | 95 |
- Beads were recovered undamaged after each use
- Single charge of catalyst avoiding multiple stage reaction
Nitro Reduction
Hydrogenation of 4-chloro-3-nitrobenzonitrile:

Ni EnCat™ (440 mg of 60 % water wet beads, 10 wt% Ni on substrate) was washed three times with IPA to remove water, decanting the wash solvent from the beads each time. 2-Chloro-5-cyano-nitrobenzene (366 mg, 2 mmol) and IPA (6 mL) were added and the reaction mixture stirred at 60°C under 10 bar hydrogen. The reaction was run for 12 hours after which time a sample was taken and the reaction analysed by GCMS. Analysis showed the formation of a single product and near quantitative (>99%) conversion of the starting material. On completion of the reaction, the beads were filtered, washed three times with IPA and the solvent removed in vacuo to give the desired product (286 mg, 1.9 mmol, 94% yield).
Reductive Amination
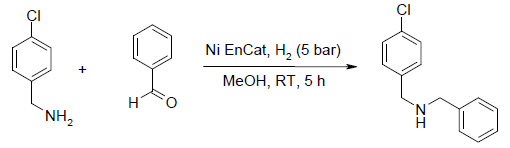
Ni EnCat™ (146 mg of 60 % water wet beads) was washed three times with methanol to remove water, decanting the wash solvent from the beads each time. Benzaldehyde (102 µl, 1 mmol), 4-chlorobenzylamine (122 µl, 1 mmol) and MeOH (4 mL) were added and the reaction was run for 5 hours at r.t (25 °C) at 5 bar after which time a sample was taken and the reaction analysed by GCMS. Analysis showed complete consumption of the benzaldehyde starting material and a 95% conversion to the desired product.
Aldehyde / Ketone Reduction
Hydrogenation of benzophenone:

Ni EnCat™ (874 mg of 60 % water wet beads) was added to the autoclave. Benzophenone (364 mg, 2 mmol) and IPA (6 mL) were added and the reaction was run for 22 hours at 60°C at 5 bar after which time a sample was taken and the reaction analysed by GCMS. Analysis showed the formation of a single product and complete consumption of the starting material. On completion of the reaction, the beads were filtered, washed three times with IPA and the solvent removed in vacuo to give the desired product.




